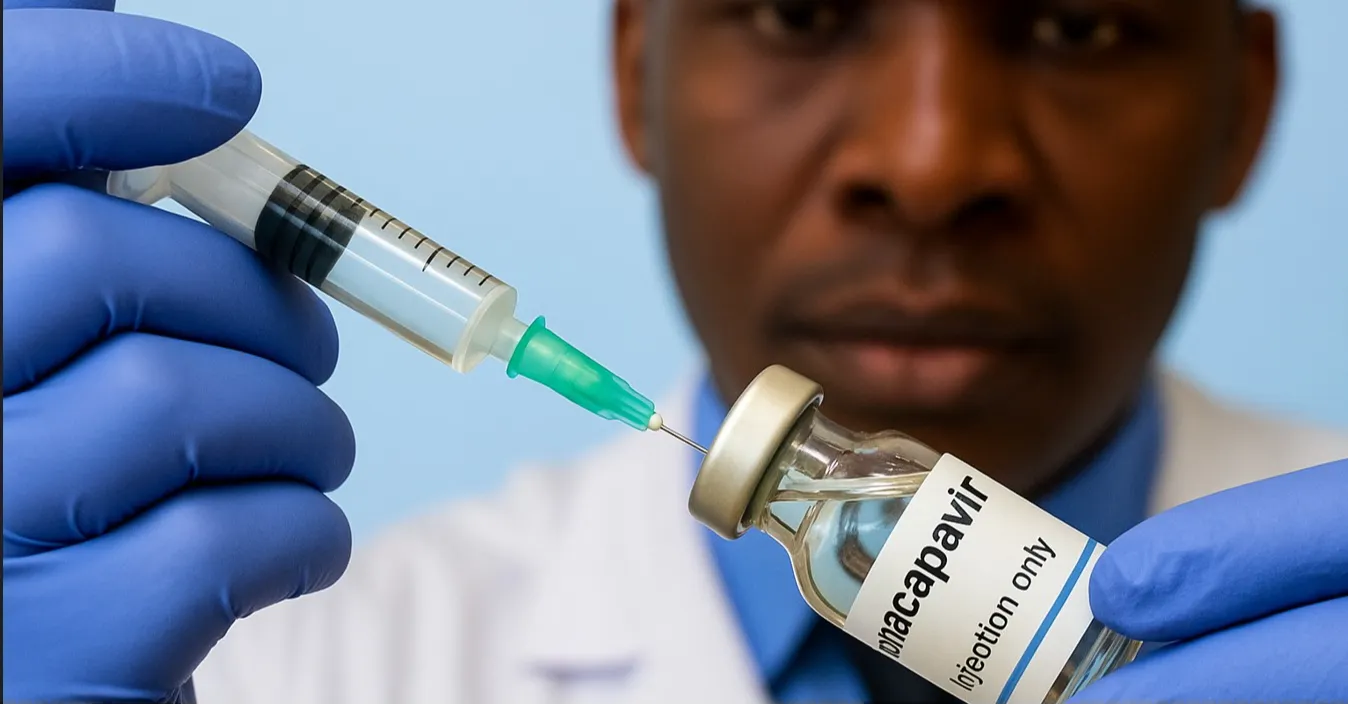
KAMPALA, Uganda — Uganda is set to receive 94,560 doses of the long-acting HIV prevention injectable drug Lenacapavir next week, marking a major step in expanding the country’s HIV prevention toolkit.
Health authorities say the consignment, already in transit, will be delivered to the country’s central medical supply systems ahead of a national rollout scheduled to begin in March.
The twice-yearly injection is expected to benefit tens of thousands of people in populations considered most vulnerable to new HIV infections.
Lenacapavir, developed by the US pharmaceutical company Gilead Sciences, has drawn global attention after early studies indicated near-complete protection against HIV acquisition, positioning it as one of the most promising biomedical prevention tools in recent years.
Of the incoming doses, 34,560 have been financed through the Global Fund, while 60,000 were donated by the United States government.
The supply is expected to cover approximately 47,280 people for one year under the initial rollout phase.
Priority groups include adolescent girls and young women aged 15–24, pregnant and breastfeeding mothers, sex workers and their clients, fishermen, long-distance truck drivers and individuals with multiple sexual partnerships, demographics that account for a disproportionate share of new infections.
Health officials say the injection will initially be available in roughly 300 public and faith-based health facilities across the country as part of the national pre-exposure prophylaxis (PrEP) programme.
Uganda continues to face a significant HIV burden, with tens of thousands of new infections recorded annually and young women remaining among the most affected groups.
Also Read: Kenya rolls out twice-yearly HIV prevention injection lenacapavir
While national prevalence has gradually declined, public health experts say innovative prevention methods are essential to accelerate progress toward epidemic control.
Lenacapavir is administered only twice a year, a feature experts believe could improve adherence compared with daily oral PrEP pills and reduce stigma linked to HIV prevention medication.
Globally, the drug has been described as a potential “game-changer,” with pilot deliveries already underway in several African countries under initiatives supported by international donors and partners.
The rollout in Uganda comes as other African countries begin introducing long-acting HIV prevention injections, reflecting a broader shift toward biomedical prevention strategies that combine behavioural, structural and pharmaceutical approaches.
Public health advocates say sustained funding, regulatory approvals and supply expansion will be critical to ensure equitable access as demand grows across high-burden regions.